
Published on-line and in print in R&D magazine. See rdmag.com/article/2019/01/r-d-efforts-make-plastic-more-sustainable


A few large facilities make plastic, dispersed through converters and suppliers, which ultimately ends up in the hands of consumers. Tens of production sites feed hundreds of converters supplying thousands of stores shopped by millions of consumers. Virtually everyone in a modern society touches plastic almost every minute of every day. We now recognize that our use patterns create environmental plastic in designed use, like rolling tires over roadways or washing clothes, and in unintended use, like escape of plastic trash from the waste system. Providing solutions is an area of increasing research focus.
Polyethylene and polypropylene are the two largest volume plastics, both commonly used in single-use packaging. By volume, the next largest resin used in packaging is polyethylene terephthalate, PET, used in beverage bottles and clear containers. Polyethylene is used as high-density resin for rigid applications like milk and detergent bottles, and also as flexible film. Bags and films are high, low or linear-low density polyethylene. Studies continue to show that the overall environmental impact of plastic bags is far less than the alternatives. Disposed of properly and kept out of the environment, plastic is the best choice for many applications. Compelling properties drive plastic use. It is flexible, robust, long-lasting and inexpensive. Concerns about environmental plastic led to bans on bags, straws and increased scrutiny of all single-use plastic items. One count now has 349 U.S. cities, counties and states with regulation either banning or taxing plastic bags alone. The plastics industry is responding, as we always do, with technology. The industry is going several different directions and trying a variety of different solutions to reduce the impact of plastic on the planet. Industry efforts to reduce the impact fall into three broad categories: use less, recycle more and reduce the impact of what escapes to the environment.
Use less plastic
Catalyst scientists, materials scientists and packaging designers continue to push boundaries, improving the performance to allow more to be done with less. Today’s resins are much stronger than previous generation materials, letting a thinner film do the same job that would have required a thicker film in the past. In many applications, resin use is cut substantially. Water bottles are one place where the reduction in resin used is obvious. Today’s bottles are substantially thinner, requiring far less resin, more than 50 percent less than previous generations.
Advances in polymers frequently go unnoticed. There are exceptions, such as R&D 100Award winning INNATE™ Precision Packaging Resin. This bimodal polyethylene gives stiff, tough and clear films with improved mechanical properties. Compared to traditional polyethylenes, it achieves up to 80 percent less haze, twice the impact strength, twice the tensile modulus, three times the puncture resistance, and three times the tensile strength. Down-gaging, using thinner films, with INNATE™ or other high performance polymers reduces overall plastic use.
Replacement reduces packaging used. A trip to any grocery store illustrates how flexible packaging is replacing thicker, heavier rigid packaging for many products. Wine, oil, paint, ketchup, nuts, and soap are just some examples where packaging is shifting away from ridged to flexible. Material replacement leads to significant reduction in material use, and, with it, reduction of fuel used to transport goods both through reduced weight and more efficient packing of trucks.
Recycle more
There are two major paths to recycling, mechanical and chemical. Mechanical recycling is reprocessing scrap material, using the scrap instead of or added to virgin resin. Most resins degrade slightly during processing. Use of post-consumer material requires sorting and cleaning. Separation of mixed polymer streams is challenging. Contamination concerns loom large, limiting recycled polymer in food contact applications. Mechanical recycling of consumer material back to original or higher value use largely remains an aspirational goal, with much recycled material going to lower-value use. Chemical recycling describes methods that converts plastics to monomers, enabling the production of virgin polymer again without fear of contamination. Chemical recycling recycles produces material suitable for original or higher value use.
Research to improve mechanical recycling continues to make great strides. The 2017 Newcomb Cleveland Prize was awarded for work synthesis of multi-block copolymers with tailorable blocks that serve as compatibilizers for blends of polyethylene and polypropylene. While very similar, they are immiscible in a melt and limit recycling. A recognized issue, there are a variety of products already on the market. ExxonMobil produces Vistamaxx™ performance polymers and Dow produces INTUNE™ Olefin Block Copolymers, to name two. INTUNE™ Olefin Block Copolymers are made using novel chain-shuttling catalysis that overcomes limitations of the living polymerization recognized with the Newcomb prize, polymerizing ethylene and propylene in a robust industrial process. Research will certainly continue to refine and improve compatibilizers added to facilitate recycling.
Making materials more easily recycled also is an area of active innovation. Compatibilzers can be added into the virgin material making it better for recycling. Most users are unaware of the technical complexity contained in the packaging they encounter. Many packages are made up of multiple layers, with layers for structure, for barrier and for aesthetics. Multiple materials complicate recycling so efforts to provide more functionality from the same base resins continue to be ripe for innovation. Dow introduced RecycleReady™ Technology that uses a range of polyethylene resins to replace difficult to recycle resins in multilayer structures, improving recyclability.
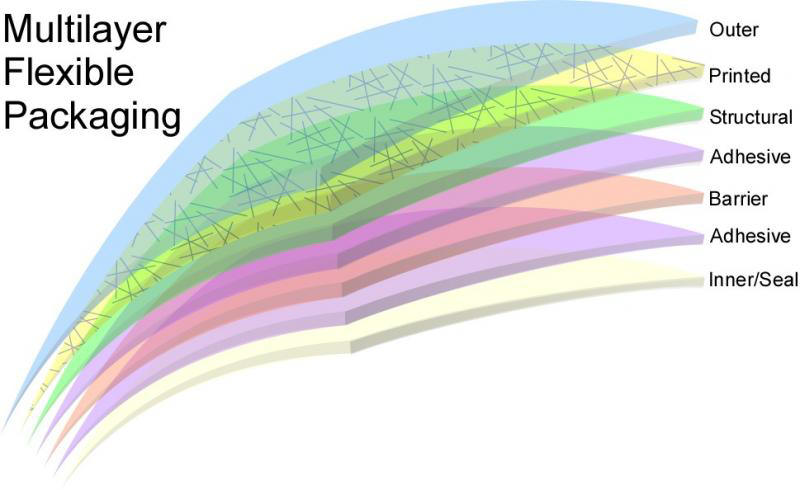
There will always be difficult to recycle materials, that are contaminated or difficult to separate. Coated paper cartons and boxes, where polymers are tightly adhered, present insurmountable challenges in polymer recycling. Processing the polymers for energy content is one option for keeping these materials out of the environment. Additional processing can turn the polymers into liquid fuels or the polymers can be used directly in energy production. Successful pilot efforts proved that community recovery is possible and a growing number of municipalities are pursuing options for energy production.
Chemical recycling
Chemical recycling is in its infancy when compared to mechanical recycling. Depolymerizing plastics back to monomers and using those monomers to make virgin material is more energy intensive than mechanical recycling, but allows polymer producers to regain control over the end product properties, producing the highest value products. Technology can be polymer dependent or completely agnostic to the feed composition.
Loop Industries is representative of a chemical route specific to a single polymer. Their process depolymerizes polyethylene terephthalate (PET) into dimethyl terephthalate and ethylene glycol in a catalytic process, and, after purification, produces PET again. The PET produced is suitable for any and all uses of virgin PET, including food and water contact. This process is not commercial on a wide scale and there are competing processes. Others are proposing new polymer systems that are more easily depolymerized, but introducing a new-to-the-world polymer is difficult.
The polyolefin resins are not well suited for depolymerization. There are no reports of high yield back to the monomers. The current options for chemical recycling involve gasification of the polymers, as pure plastic or as a plastic-containing municipal solid waste stream, to form synthesis gas or pyrolysis to make an oil suitable for steam cracking after additional processing. Synthesis gas can be converted to methanol, ethanol, or naphtha for subsequent processing to ethylene and/or propylene. Olefins are produced, albeit through a complex pathway, that are identical to the olefin feedstocks used in the production of the virgin resin. Chemical recycling is in its infancy and is less economical than production from virgin feedstock, due largely to high capital. Technology improvements are clearly needed and likely attainable.
Reducing impact
Some slip of polymers to the environment is likely inevitable. Reducing the impact of polymers that escape into the environment means shortening the lifetime in the environment. Polyethylene, polypropylene and PET are widely used because they are robust, resilient and largely immune from biodegradation. If released, they can persist for years. Development of new materials or modification of existing polymers to speed degradation reduces impact.
Many bidegradable polymers are known, yet none have managed to compete with polyethylene, polypropylene and PET. Research efforts to develop better biodegradable and environmentally degradable polymers continues. The robust and mature recycling infrastructure hinders new polymer introduction. Inroads are being made were the waste can be controlled, such as in stadiums specifying only compostable containers and food service items. New polymers must be compatible with the existing recycling infrastructure for use more broadly.
One of the more tantalizing, though largely unrealized, options is modification of conventional resins l to degrade in the environment. Methods employing biological fillers, like starch, are problematic. These lead to visual reduction of litter when the filler degrades leaving only small particles of plastic. Recent focus on persistent microplastic particles calls into question the wisdom of such approaches. Additives that improve environmental oxidation of polyethylene are not widely used. Concern over contamination and degradation of the recycle stream remains an issue. Newer research is changing perceptions of what is possible. Enzymatic degradation of polyethylene is now proven, providing new avenues of exploration. Additives that render polyethylene biodegradable are now on the market. New discoveries hold the promise of making conventional polymers decompose in the environment.
Cleaner future
Reducing the amount of plastic in the environment is an active research area. Improving plastics so that less is used, developing methods to recycle more and harnessing nature to reduce the impact of what escapes to the environment all are rich areas of study. Societal pressure and willingness to support new options will ultimately determine what is succeeds. What is clear is that the R&D community is providing new options for a cleaner, more circular future.
Captured for mjphd.net on 19 July 2019.